You never miss your water...
 Throughout history great beers have been made in many different parts of the world. Many different factors have played a role on the development and evolution of brewing traditions and practices around the world.
Throughout history great beers have been made in many different parts of the world. Many different factors have played a role on the development and evolution of brewing traditions and practices around the world.
Beer is an agriculturally derived product, so the specific characteristics of beer have historically varied from region to region depending upon the particular growing circumstances associated with a given region. Beer characteristics have also been affected by differing regional political influences on taxation and trade; tax rates and ingredient availability certainly played a role in the brewer’s choice to produce a particular kind of beer.
Beer characteristics are also closely linked the particular methods of malting and kilning of the barley that were employed in a given region of the world. The characteristics of beer are also very much influenced by every step of the process of manufacturing of beer, and has been impacted greatly by the common brewing practices that have been traditionally employed in different parts of the world.
Over time, different cities have become famous for the production of particular styles of beer. There are many reasons for this, but perhaps the greatest influence on the reason that a particular beer style has come to be associated with a particular city is the brewing water.
Water Chemistry Profiles of Famous Brewing Cities
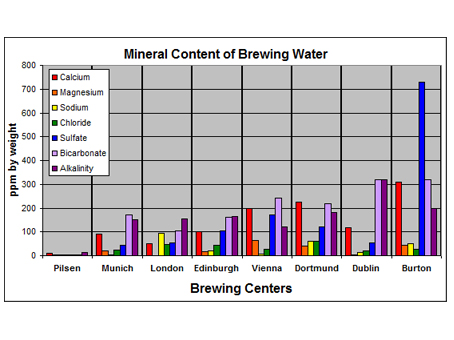
Brewing operations around the world were established long before the chemistry of water was understood. Brewers learned from experience that a brewing recipe that might produce favourable results in one city, might not produce good results in a different city. Beer styles evolved to suit the water of the city in which they were brewed. A brewer grew to understand what types of beer were well suited to the water that was available for brewing. Water profiles of several of major brewing cities are shown in Table 1 and in Figure 1.
Table 1: Water profiles for classic brewing cities (concentration in ppm)
[table id=11 /]
Figure 1: Water profiles for classic brewing cities (concentration in ppm)
It is clear from the data that water naturally occurring in different cities has very varying levels of dissolved minerals. It is these differences that have helped define the beer and brewing of these cities. Here are descriptions of the distinct brewing waters associated with different cities, and the types of beer that have come to be associated with these cities as a result of the brewing water.
Pilsen – Very soft, low-alkalinity water is optimal for the pale color and clean bitterness of a Pilsner beer. The soft water allows the proper mash pH to be reached using only base malts, and helps the beer achieve the softly-rich flavour of fresh bread. The low sulfate content enables mellow hop bitterness without overpowering the soft maltiness. It also enhances noble hop aroma.
Dortmund – Dortmund is famous for the production of pale lagers and specifically famous for Dortmund Export. Dortmund Export has less hop character than a Pilsner, but a more assertive malt character that is enhanced by the higher levels of all minerals. The balance of the minerals is similar to Vienna, but the beer is bolder, drier, and lighter in colour. Dortmund water is considered to be relatively hard water, with fairly high levels of most all of the water minerals. This water is ideal for making the medium-bitter, pale style that is the Dortmund Export lager.
Vienna – Vienna is known for the production of well-balanced, amber lagers. Vienna has water that contains low levels of sodium and chloride but has a relatively high level of overall hardness. It also has a relatively high sulfate content that helps contribute to a dry finish and can help accentuate the nuttiness of the slightly more highly-kilned malts that are associated with this kind of beer.
Munich – Munich is a city that is known for its dunkles, bocks, märzens and Oktoberfest. The smooth flavours of these types of beer generally have a distinctively sweeter, malty profile that results from effectively using darker malts to balance the acidifying characteristics associated with the carbonate in the mashing and brewing waters. The relatively low sulphur content of Munich’s water enables hop bitterness to be mellow and allows the malt to dominate the flavour profile.
London – London is historically known for its bitters, milds and porters. London water varies from district to district but is exemplified by low calcium, moderate carbonates, high sodium and high chloride content. Water with moderate carbonates and high levels of sodium and chloride are well-suited for brewing smooth, dark, balanced beers that are exemplified by styles such as milds and porters. The sodium also accentuated the sweetness of porter.
Edinburgh – Edinburgh is famous for Scottish ale. This style of beer is a ruby-red, dark, sweet, malty beer with a mellow, slight hop finish. The water of Edinburgh is very similar to that of London, but has a slightly higher bicarbonate and sulfate content. This type of water is ideal for a beer with lots of malt body and enables the beer to be perceived as balanced even though only a small amount of hops are used in the brewing process.
Burton-on-Trent – Burton-on-Trent is known for India pale ale. The water of Burton-on-Trent is very high in calcium and sulfates and relatively low in sodium. The high level of sulfate and low sodium level is ideal for the production of a beer with assertive hop bitterness like an India Pale Ale.
Dublin – Dublin is famous for dry stouts. Dublin water has the highest concentration of bicarbonates of any of the famous brewing centers within the British Isles. This extremely high bicarbonate content is perfect for producing beers that require the use of the highly acidic, dark, roasted malts that define the stout style. Relatively low levels of sodium, chloride, and sulfate allow hop bitterness to unobtrusively balance all of the roasty goodness.
How Dissolved Substances Influence Beer
All brewing water contains dissolved ions that influence various steps in the brewing process and have an impact on the final flavor of the finished beer. Adjusting the concentrations of these substances allows a brewer to customize the brewing water to match that of a historical brewing centre, or to produce brewing water that is ideally suited for the production of a beer with particular characteristics. Table 2 below provides an overview of how various dissolved ions impact beer.
Table 2: How dissolved ions impact beer
[table id=12 /]
Making Great Beer
Great beer is made from great ingredients. Since beer is mostly made of water, it is not surprising that the composition of the water is very important to taste and characteristics of the finisher beer. By matching the mineral content of your brewing water to that of the city from which a particular beer style originated, you can be sure that your beer will be as authentic as possible.
To obtain a desired mineral content in your brewing water, obtain an assay of your brewing water and then make adjustments based on the information given in Table 3.
Table 3: Contribution of various substances to brewing water
[table id=13 /]
References:
1) Freccia, Nico, Brew Your Own, May 1996
2) Palmer, John, How to Brew, Chapters 4 & 15, 2006, p.37-39, 153-165
Links About Brewing Water:
http://www.antiochsudsuckers.com/tom/brewingwater.htm
http://www.homebrew.com/mike_brew_corner/mike_03270101.shtml
http://www.brodiescastlebrewing.com/index.php?topic=142.0
http://www.howtobrew.com/section3/chapter15-2.html.
[adrotate group=”1″]