Beer bottles: The answer is not clear

An inviting image but a well-hopped beer in clear glass can become noticeably offensive in just 30 seconds.
Beer is a beautiful and complex drink. Several hundred different chemical compounds have been identified within a typical beer. Of these myriad compounds, those derived from hop additions during the brewing process may, perhaps, be the most important.
Compounds derived from hops are vital to the organoleptic qualities of beer, and play several roles, but in particular they are crucial as a source of aroma (from the essential oils) and bitterness (from the hop resins).
Although the flavour and aroma chemistry associated with hop oils and hop polyphenols is rather complex, the chemistry of the compounds associated with the bitter taste features of beer are well understood. The most important compounds associated with hop-derived bitterness are the α-acids (alpha acids).
How alpha acids get into beer
In a pure state, the hop-acids are weak acids that occur as pale-yellowish solids. The naturally occurring α-acids exhibit very poor solubility in water and have almost no bitter taste. α-acids can account for between 2 and 15 per cent of the dry weight of the hop, depending upon the specific hop variety and the hop storage environment. Hops with higher α-acid content have greater bittering potential.
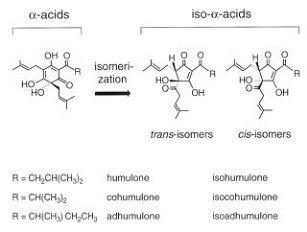
When energy is applied to these molecules during the boiling of the wort, the atoms within the α-acid molecules rearrange themselves and sort of “open up”. The α-acid molecules are isomerized to form iso-α-acids. There are three different α-acids in hops. These α-acids are molecularly similar, but differ from one another in their side-chain structure. Each iso-α-acid exists in two forms, cis and trans, which differ in the orientation of the side chains (the “-R” groups shown in Figure 1) relative to the rest of the components within the molecule. The six iso-α-acids differ in the quality and intensity of their bitterness. The isomerization reaction of α-acids to iso-α-acids is shown in Figure 1.
Beer bitterness from hops is due to the presence of iso-α-acids in concentrations that typically vary between 15 and 100 parts per million within the beer (ppm). One part per million is equivalent to one drop of water diluted into 50 liters.
The photochemical effect of light on beer
The flavour of beer is directly related to the ingredients used to make the beer. Beer flavour is also affected by the details associated with the brewing process.
“Beer flavour continues to change after packaging and storage, and can be dramatically altered by exposure to heat and light.”
Beer flavour continues to change after packaging and storage, and can be dramatically altered by exposure to heat and light. Exposure to heat will increase the oxidation rate of the compounds in beer and produce a flavour and aroma that is often described as “wet paper”, “cardboard” or “sherry-like”. Exposure to light will produce offensive flavours and aromas that are described as “catty”, “skunk-like” or “lightstruck”.
There are many opportunities for beer to be exposed to light during storage. Beer is exposed to light when sitting on a display shelf or inside the refrigerator at your local bottleshop. It is also exposed to light inside your refrigerator at home. Beer may be exposed to sunlight if allowed to sit outside.
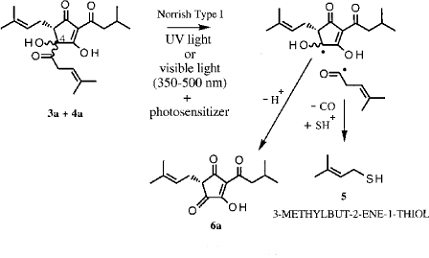
Figure 2: When light hits beer, it provides the energy necessary to drive the chemical reaction that causes light strike
When light hits beer, it provides the energy necessary to drive a chemical reaction (see Figure 2) that transforms the iso-α-acids into 3-methylbut-2-ene-1-thiol. The “thiol” part of the name indicates that there is sulfur present. Sulfur compounds often have strong, offensive aromas. The flavour threshold of 3-methylbut-2-ene-1-thiol is so low that a concentration of even a few parts per billion (equivalent to one drop in 50 000 litres) is enough to irreversibly spoil the beer and impart the characteristic “lightstruck” flavours and odours.
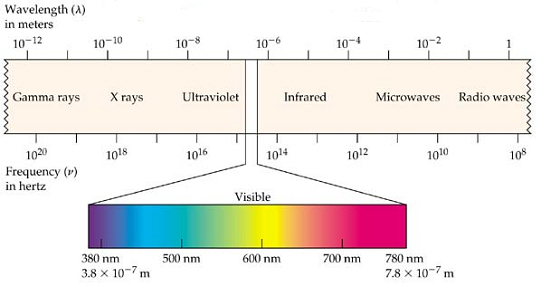
The detrimental impact of light on beer is greater for higher energy light. Higher energy light has a shorter wavelength. Light with a wavelength of 380 nanometres (nm) is higher in energy than light with a wavelength of 500 nm, and 500 nm light is higher in energy than 600 nm. High-energy light is usually associated with ultraviolet (UV) light that has a wavelength of 280 to 380 nm. Figure 3 shows the wavelength of various colours of light and other types of electromagnetic radiation.
How packaging protects beer from lightstrike
Beer can be entirely protected from being “lightstruck” by storing it in opaque containers such as cans or kegs. Beer that is packaged and stored in bottles is susceptible to developing lightstruck flavours.
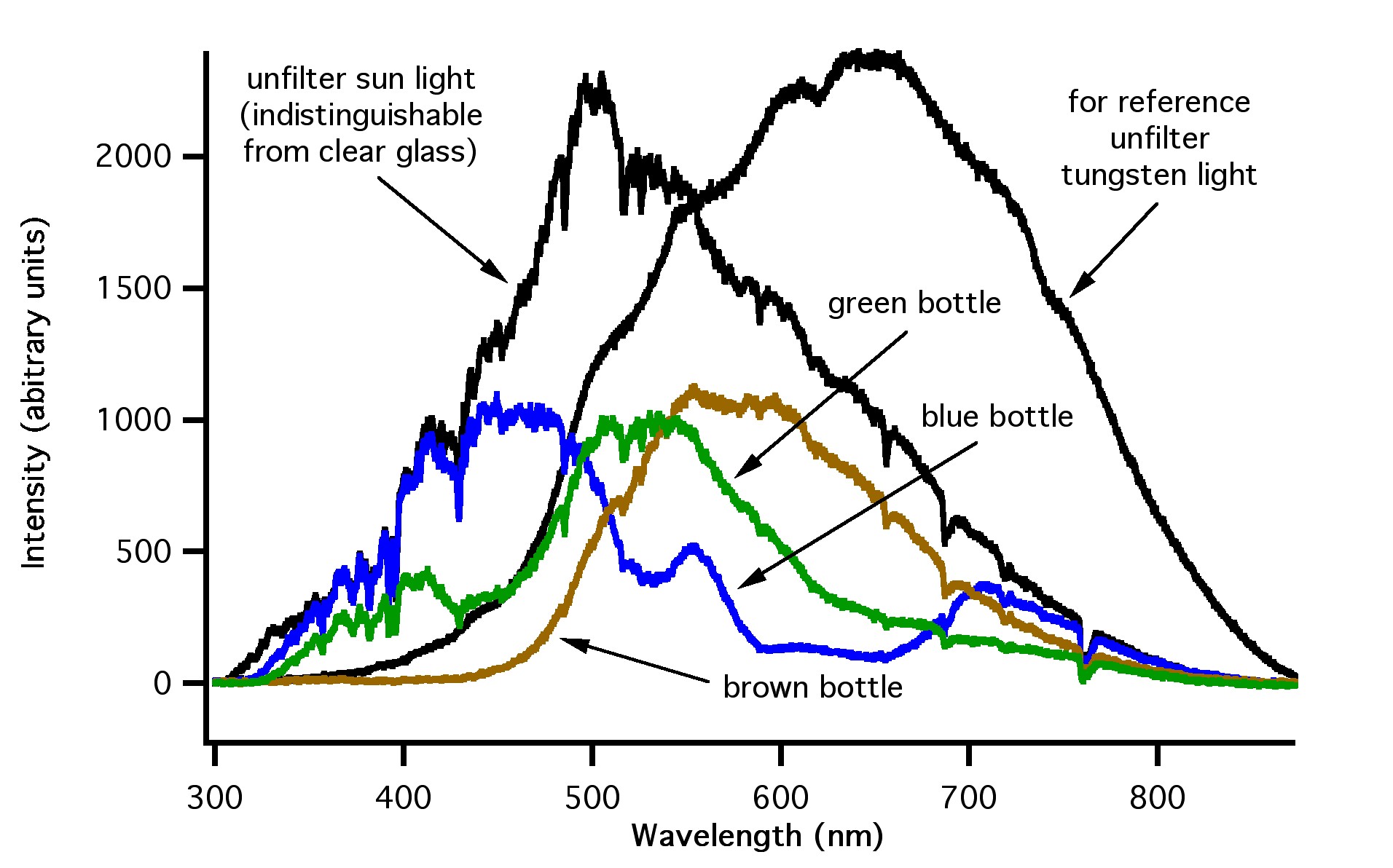
The level of protection provided by the bottle depends upon the colour of the glass because the wavelengths of light that are absorbed or transmitted through glass are also dependent upon the colour of the glass. Figure 4 shows how light emitted from a standard, tungsten-filament light bulb is filtered by clear, green, blue and brown glass. Figure 5 shows the same thing for sunlight, and Figure 6 shows it for fluorescent bulbs.
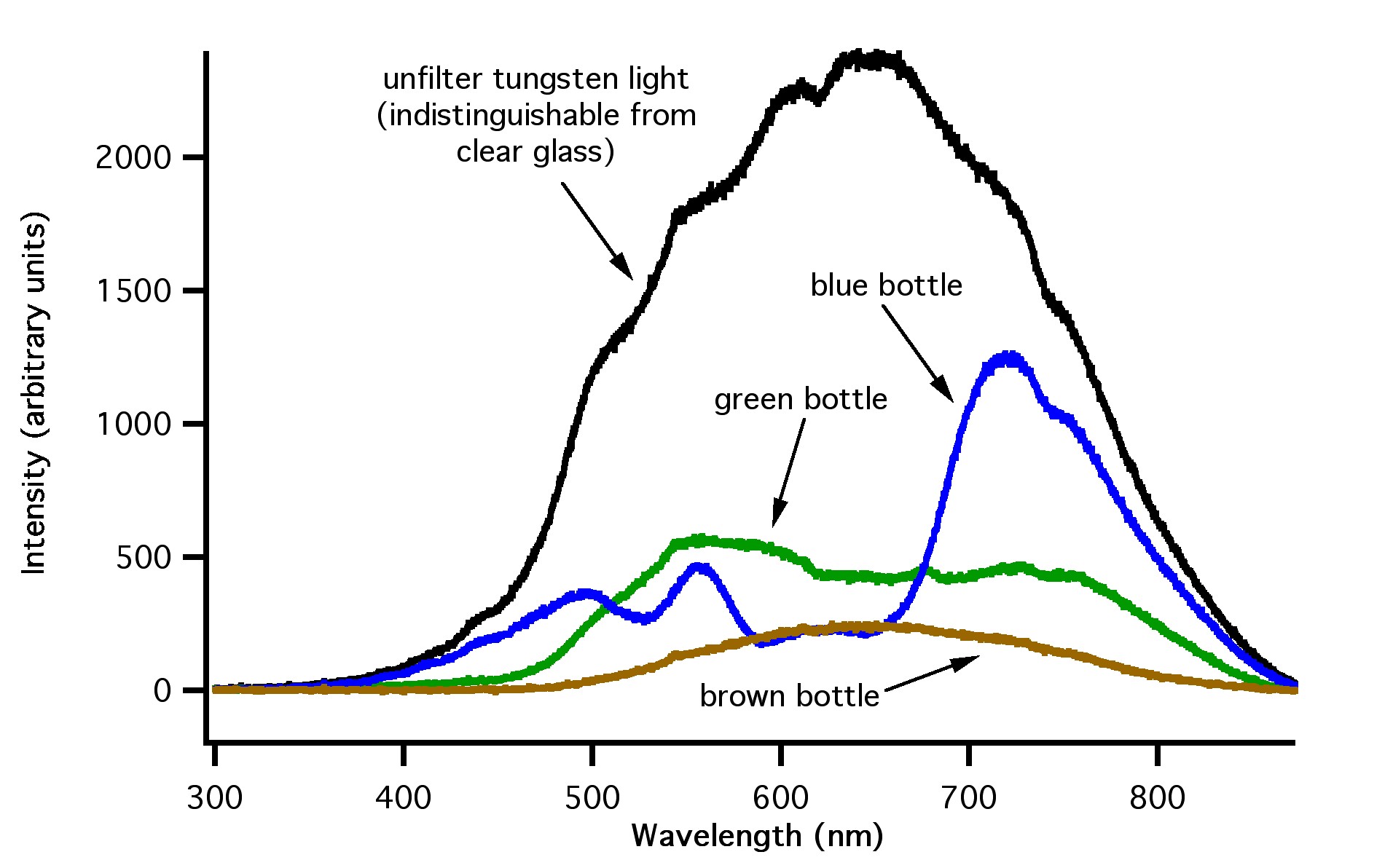
Figure 4: How light emitted from a standard, tungsten-filament light bulb is filtered by clear, green, blue and brown glass.
Figure 4: Emission spectra from standard tungsten light bulb (black line), the standard tungsten light bulb filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Note the visible region is from ~400 to 700 nm, ultraviolet region is ~ 280 to 380 nm, and the infrared region is greater than ~700 nm.
Figure 5: Emission spectra from the sun (black line – left), standard tungsten light bulb (black line – right), the sun filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Note the sun produces significantly more ultraviolet light when compared to the tungsten bulb.
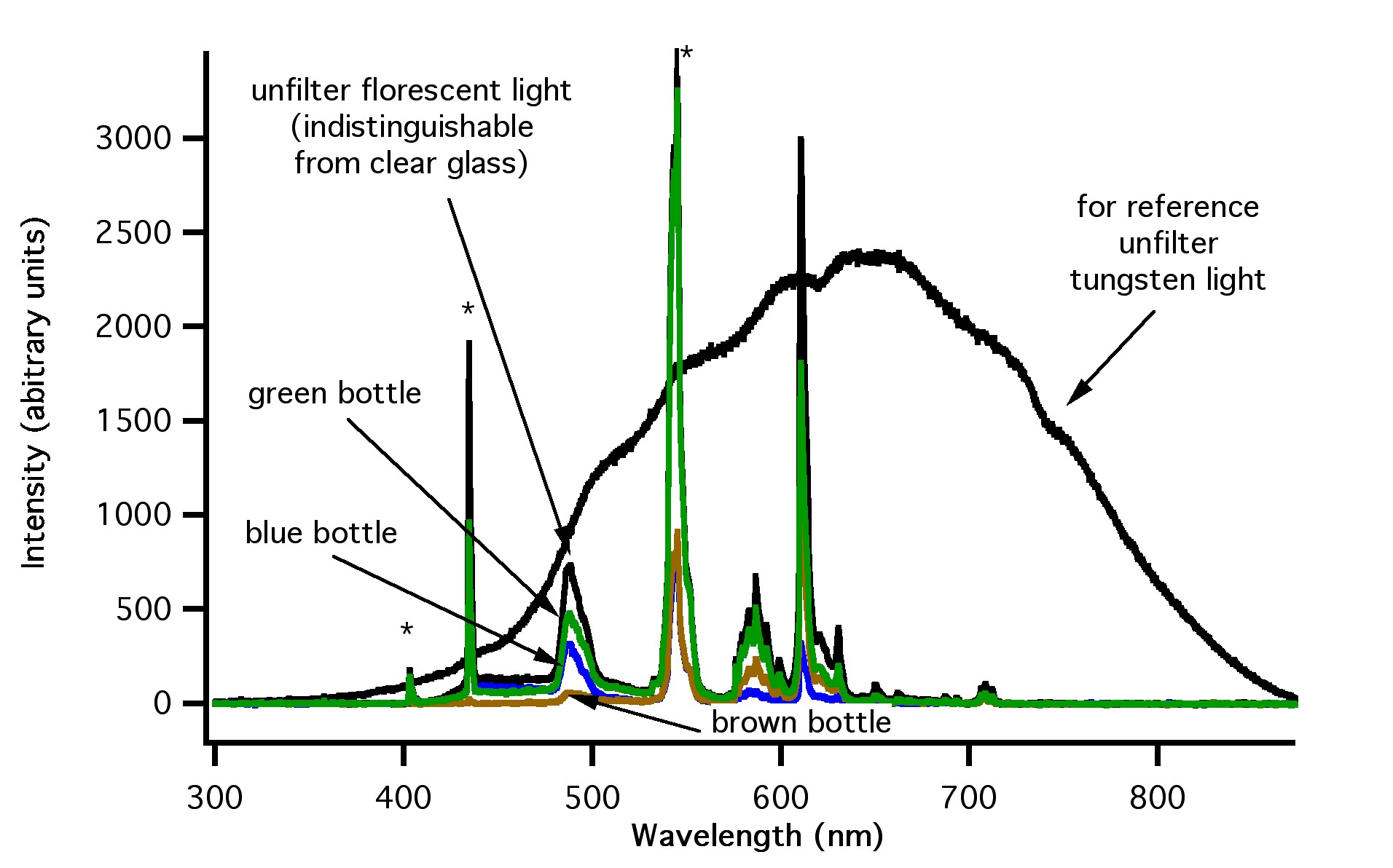
Figure 6: How light emitted from a fluorescent bulb is filtered by clear, green, blue and brown glass.
Figure 6: Emission spectra from a standard fluorescent tube (black line – sharp), standard tungsten light bulb (black line – broad), fluorescent light filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Emission lines from mercury are labeled with an asterisk
Note that the emission spectra from the three sources are very different. The sun’s spectrum is shifted to higher-energy, shorter wavelengths compared to the tungsten bulb, while the fluorescent bulb has well defined emission line “spikes” due to the fact that fluorescent bulbs work based on the excitation of mercury vapour.
The data presented in Figures 4, 5 and 6 show that brown glass filters the largest amount of high energy light, while blue, green and clear glass filters much less. Note that green and blue glass is much less effective than brown glass for filtering the higher energy UV light (less than ~ 400 nm).
The photochemical reaction occurs very quickly; a well-hopped beer in clear glass can become noticeably offensive with just 30 seconds of exposure to sunshine.
Another approach at protecting beer from lightstrike: chemically altering the iso-α-acids
Apart from storing beer in light-proof containers, the photosensitivity of beer can be reduced by chemically altering the iso-α-acids so that the chemical precursor to the photochemical reaction responsible for producing the lightstruck flavour is not present within the beer.
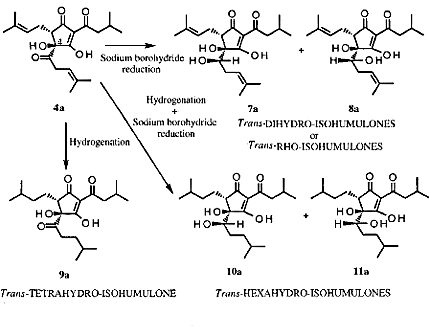
Iso-α-acids can be converted to reduced iso-α-acids by hydrogenation and/or by reaction with sodium borohydride. Three major types of reduced iso-α-acids can be produced: dihydro, tetrahydro, and hexahydro. Figure 7 shows the reactions.
Although the chemically reduced iso-α-acids are as photoreactive as the ordinary iso-α-acids, 3-methylbut-2-ene-1-thiol cannot be formed from these compounds subsequent to photocleavage. As a result, the lightstruck flavour developed from reduced iso-α-acids has distinctly different, and much less obnoxious, organoleptic features than the lightstruck flavour resulting from photocleavage of the ordinary iso-α-acids. Substitution of iso-α-acids by dihydro, tetrahydro and hexahydro-iso-α-acids allows brewing of light-stable beers, which can be bottled in clear or green glass. Additionally, the bittering properties of dihydro, tetrahydro and hexahydro-iso-α-acids are comparable to that of ordinary iso-α-acids as shown in Table 1.
[table id=1 /]
Conclusions
The best way to keep beer from having lightstruck flavours and odours is to prevent it from coming into contact with light. Packaging beer in opaque containers such as cans or kegs will guarantee that no photochemical reactions occur. If beer is to be packaged in bottles, then brown bottles are definitely the best choice. If beer must be packaged in bottles that are green or clear, then the use of chemically reduced iso-α-acids can allow the brewer to produce a beer that remains light-stable.
References
Burns, C.S., Heyerick, A., De Keukeleire Denis, and Forbes, M., Mechanism for Formation of the Lightstruck Flavor in Beer Revealed by Time-Resolved Electron Paramagnetic Resonance, Chem. Eur. J., 2001, 7(21)
De Keukeleire, Denis, Fundamentals of Beer and Hop Chemistry, Quím. Nova vol.23 n.1 São Paulo Jan./Feb. 2000
de Piro, George, Beer Flavors 2: Skunkiness, http://www.evansale.com/skunked beer.html
Pozdrik, R., Roddick, F., Rogers, P., and Nguyen, T., Spectrophotometric Method for Exploring 3-Methyl-2-butene-1-thiol (MBT) Formation in Lager, J. Agric. Food Chem, 2006, 54(17)
Sturgeon, Bradley E., Light Absorption by Various Beer Bottle Glass: Data to Accompany April 2nd, 2008 Basic Brewing Radio Podcast
[adrotate group=”1″]
Beer bottles: The answer is not clear
Beer is a beautiful and complex drink. Several hundred different chemical compounds have been identified within a typical beer. Of these myriad compounds, those derived from hop additions during the brewing process may, perhaps, be the most important.
Compounds derived from hops are vital to the organoleptic qualities of beer, and play several roles, but in particular they are crucial as a source of aroma (from the essential oils) and bitterness (from the hop resins).
Although the flavor and aroma chemistry associated with hop oils and hop polyphenols is rather complex, the chemistry of the compounds associated with the bitter-taste features of beer are well understood.The most important compounds associated with hop-derived bitterness are the a-acids (alpha acids).In a pure state, the hop a-acids are weak acids that occur as pale-yellowish solids.The naturally occurring a-acids exhibit very poor solubility in water and have almost no bitter taste.a-acids can account for between 2 and 15% of the dry weight of the hop, depending upon the specific hop variety and the hop storage environment. Hops with higher a-acid content have greater bittering potential.
When energy is applied to these molecules during the boiling of the wort, the atoms within the a-acid molecules rearrange themselves and sort of “open up”.The a-acid molecules are isomerized to form iso-a-acids. There are three different a-acids in hops.These a-acids are molecularly similar, but differ from one another in their side-chain structure. Each iso-a-acid exists in two forms, cis and trans, which differ in the orientation of the side chains (the “-R” groups shown in Figure 1) relative to the rest of the components within the molecule. The six iso-a-acids differ in the quality and intensity of their bitterness. The isomerization reaction of a-acids to iso-a-acids is shown in Figure 1.
Beer bitterness from hops is due to the presence of iso-a-acidsin concentrations that typically vary between 15 and 100 ppm within the beer.
The photochemical effect of light on beer
The flavor of beer is directly related to the ingredients used to make the beer.Beer flavor is also affected by the details associated with the brewing process.Beer flavor continues to change after packaging and storage, and can be dramatically altered by exposure to heat and light.Exposure to heat will increase the oxidation rate of the compounds in beer and produce a flavor and aroma that is often described as “wet paper”, “cardboard” or “sherry-like”.Exposure to light will produce offensive flavors and aromas that are described as “catty”, “skunk-like” or “lightstruck”.
There are many opportunities for beer to be exposed to light during storage.Beer is exposed to light when sitting on a display shelf or inside the refrigerator at your local bottleshop. It is also exposed to light inside your refrigerator at home.Beer may be exposed to sunlight if allowed to sit outside.
When light hits beer, it provides the energy necessary to drive a chemical reaction (see Figure 2) that transforms the iso-a-acids into 3-methylbut-2-ene-1-thiol.The “thiol” part of the name indicates that there is sulfur present. Sulfur compounds often have strong, offensive aromas. The flavour threshold of 3-methylbut-2-ene-1-thiol is so low that a concentration of even a few parts-per-billion is enough to irreversibly spoil the beer and impart the characteristic “lightstruck” flavors and odors.
The detrimental impact of light on beer is greater for higher energy light.Higher energy light has a shorter wavelength.Light with a wavelength of 380 nm is higher in energy than light with a wavelength of 500 nm, and 500 nm light is higher in energy than 600 nm.High-energy light is usually associated with ultraviolet (UV) light that has a wavelength of 280 to 380 nm.Figure 3 shows the wavelength of various colours of light and other types of electromagnetic radiation.
How packaging protects beer from lightstrike
Beer can be entirely protected from being “lightstruck” by storing it in opaque containers such as cans or kegs.Beer that is packaged and stored in bottles is susceptible to developing lightstruck flavours.The level of protection provided by the bottle depends upon the colour of the glass because the wavelengths of light that are absorbed or transmitted through glass are also dependent upon the colour of the glass.Figure 4 shows how light emitted from a standard, tungsten-filament light bulb is filtered by clear, green, blue and brown glass.Figure 5 shows the same thing for sunlight, and Figure 6 shows it for fluorescent bulbs.
Figure 4: Emission spectra from standard tungsten light bulb (black line), the standard tungsten light bulb filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Note the visible region is from ~400 to 700 nm, ultraviolet region is ~ 280 to 380 nm, and the infrared region is greater than ~700 nm.
Figure 5:Emission spectra from the sun (black line – left), standard tungsten light bulb (black line – right), the sun filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Note the sun produces significantly more ultraviolet light when compared to the tungsten bulb.
Figure 6:Emission spectra from a standard fluorescent tube (black line – sharp), standard tungsten light bulb (black line – broad), fluorescent light filtered with blue glass (blue line), green glass (green line), and brown glass (brown line). Emission lines from mercury are labeled with an asterisk
Note that the emission spectra from the three sources are very different.The sun’s spectrum is shifted to higher-energy, shorter wavelengths compared to the tungsten bulb, while the fluorescent bulb has well defined emission line “spikes” due to the fact that fluorescent bulbs work based on the excitation of mercury vapour.
The data presented in Figures 4, 5 and 6 show that brown glass filters the largest amount of high energy light, while blue, green and clear glass filters much less. Note that green and blue glass is much less effective than brown glass for filtering the higher energy UV light (less than ~ 400 nm).
The photochemical reaction occurs very quickly; a well-hopped beer in clear glass can become noticeably offensive with just 30 seconds of exposure to sunshine.
Another approach at protecting beer from lightstrike: chemically altering the iso-a-acids
Apart from storing beer in light-proof containers, the photosensitivity of beer can be reduced by chemically altering the iso-a-acids so that the chemical precursor to the photochemical reaction responsible for producing the lightstruck flavor is not present within the beer.
Iso-a-acids can be converted to reduced iso-a-acids by hydrogenation and/or by reaction with sodium borohydride.Three major types of reduced iso-a-acids can be produced: dihydro, tetrahydro, and hexahydro.Figure 7 shows the reactions.
Although the chemically reduced iso-a-acids are as photoreactive as the ordinary iso-a-acids, 3-methylbut-2-ene-1-thiol cannot be formed from these compounds subsequent to photocleavage. As a result, the lightstruck flavor developed from reduced iso-a-acids has distinctly different, and much less obnoxious, organoleptic features than the lightstruck flavor resulting from photocleavage of the ordinary iso-a-acids.Substitution of iso-a-acids by dihydro, tetrahydro and hexahydro-iso-a-acids allows brewing of light-stable beers, which can be bottled in clear or green glass.Additionally, the bittering properties of dihydro, tetrahydro and hexahydro-iso-a-acids are comparable to that of ordinary iso-a-acids as shown in Table 1.
Table 1: Comparison of Bittering Properties
Compounds
Relative Bitterness
Isohumulones
1.0
Dihydro-isohumulones
0.6-0.7
Tetrahydro-isohumulones
1.5-1.9
Hexahydro-isohumulones
1.0-1.2
Conclusions
The best way to keep beer from having lightstruck flavors and odors is to prevent it from coming into contact with light.Packaging beer in opaque containers such as cans or kegs will guarantee that no photochemical reactions occur.If beer is to be packaged in bottles, then brown bottles are definitely the best choice.If beer must be packaged in bottles that are green or clear, then the use of chemically reduced iso-a-acids can allow the brewer to produce a beer that remains light-stable.
References
Burns, C.S., Heyerick, A., De Keukeleire Denis, and Forbes, M.,Mechanism for Formation of the Lightstruck Flavor in Beer Revealed by Time-Resolved Electron Paramagnetic Resonance, Chem. Eur. J., 2001, 7(21)
De Keukeleire, Denis, Fundamentals of Beer and Hop Chemistry, Quím. Novavol.23n.1São PauloJan./Feb.2000
de Piro, George, Beer Flavors 2: Skunkiness, http://www.evansale.com/skunked beer.html
Pozdrik, R., Roddick, F., Rogers, P., and Nguyen, T., Spectrophotometric Method for Exploring 3-Methyl-2-butene-1-thiol (MBT) Formation in Lager, J. Agric. Food Chem, 2006, 54(17)
Sturgeon, Bradley E., Light Absorption by Various Beer Bottle Glass: Data to Accompany April 2nd, 2008 Basic Brewing Radio Podcast